
CDSCO Audits for Class A and Class B Medical Devices
About QACA (as CDSCO notified body):
Quality Austria Central Asia (QACA) is recognized as a Notified Body by CDSCO.
QACA is approved via Notification related to MD-2 i.e. QACA can grant license for Manufacturing, Sales, Storage, Distribution and Exhibition of Medical Devices for Class A and Class B as per Schedule IV and Schedule V of MDR, 2017 as below:

About CDSCO Audits:
- Audit process covers the below stages-
- Desktop Audits;
- Onsite Audits;
- Report Writing.
QACA will do audit as per Schedule 4 (Plant Medical File i.e. MD-5) and Schedule 5 (as per ISO 13485).
The Notified Body audits (QACA) covers three broad categories during the audits onsite:
- Infrastructure
- Regulatory Compliance
- Documentation
Please note that certain MD-5 Audits are Fresh applications and some are for Endorsement (adding of newer products) which is to be applied by the clients to CDSCO accordingly.
The client applies on CDSCO Portal and uploads the following: -
- Device Medical File (DMF) – Appendix 2 – for MDD,
- Device Medical File (DMF) – Appendix 3 – for IVD,
- Plant Medical File (PMF) – Appendix 1 for IMDR 2017,
- Licenses,
- Certificates etc.
Flow Chart for CDSCO Audits:
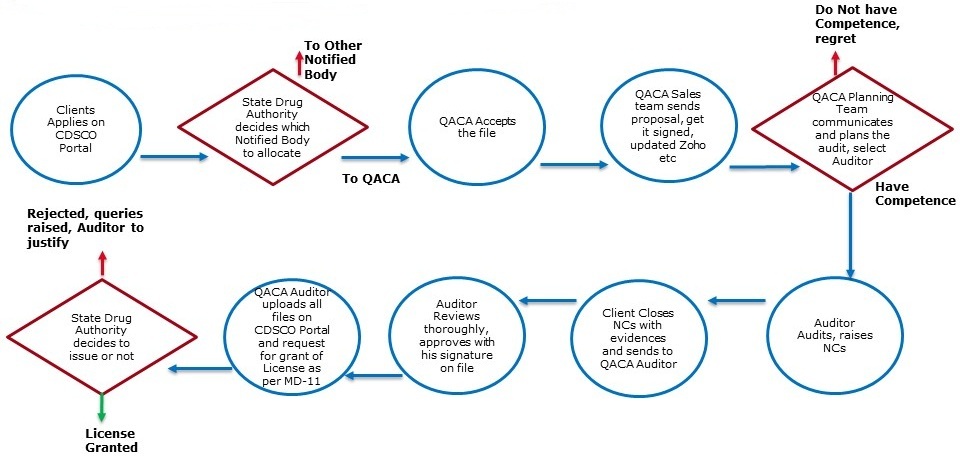
For any queries or further information related to our services, please feel free to contact us at info@qacamail.com or call us at +919599619392. We are here to assist you!





